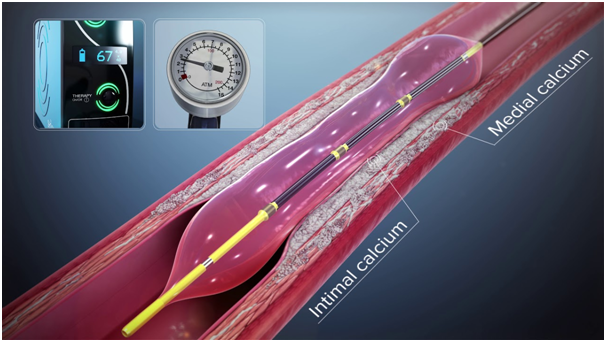
Intravascular Lithotripsy (IVL)
Coronary calcification poses a real challenge to the interventional cardiologist and is associated with a higher risk of stent underexpansion, stent thrombosis, and ischemic target lesion revascularization.
A heavily calcified lesion may be difficult to cross with a guidewire, balloon, or stent, and may prove resistant to adequate modification prior to stent implantation.
Commonly used methods for achieving this include predilatation with compliant/NC balloons, use of specialized balloons, (e.g., very high-pressure balloons, cutting balloons, scoring balloons, and rotational/orbital atherectomy and Laser angioplasty.
Intravascular lithotripsy (Shock wave-IVL) is a novel technique based on an established treatment strategy for renal calculi, in which multiple lithotripsy emitters mounted on a traditional catheter platform deliver localized pulsatile sonic pressure waves to modify vascular calcium with low incidence of complications.
Shockwave IVL delivers localized pulsatile sonic pressure waves, modifying calcified lesions in a safe and reproducible manner. The mechanism is leveraging electrohydraulic-generated lithotripsy to produce high-speed sonic pressure waves that pass through soft tissue to selectively disrupt calcium. The concept adopted is similar to urologic extracorporeal lithotripsy, but IVL differs by expressing a focal effect with ultra-high energy. IVL is designed specifically for vascular applications with the technology producing unfocused energy that creates a localized effect only within the vessel.
IVL system consists of a catheter that has the emitters and is enclosed in an integrated angioplasty balloon mounted on a rapid exchange system, shockwave generator and connector cable. The balloon should be well prepared and inflated with a saline and contrast solution without any air contained, and the size is selected according to the proximal and distal reference vessel diameter. After advancing the IVL catheter to the target lesion guided by radiopaque markers on the catheter, the balloon is inflated at 4atm to contact with vessel wall, facilitating optimal energy delivery. By pushing a button on the cable connected the catheter, the lithotripsy cycle is activated and pulses once per second for ten seconds. After every cycle, the balloon is inflated up to 6 atm, which compresses the fractured calcium. The cycle is repeated as needed until the desired lesion expansion is obtained and the maximum repeatable cycle with the same catheter is 8 cycles.
The safety and effectiveness of IVL for vessel preparation for severe coronary artery calcium in stenotic de novo lesions before stent implantation has been validated in clinical trials.
The feasibility of intravascular lithotripsy (IVL) for modification of severe coronary artery calcification (CAC) was demonstrated in the Disrupt CAD I study (Disrupt Coronary Artery Disease). We next sought to confirm the safety and effectiveness of IVL for these lesions.
The Disrupt Coronary Artery Disease (Disrupt-CAD) study is a prospective multicenter, single-arm study enrolling 60 patients with severely calcified lesions treated with shockwave coronary IVL. The main inclusion criteria of the lesions were severely calcified lesions in a native coronary artery with ≥50 percent diameter stenosis and >32 mm length. The primary performance endpoint was clinical success defined as a residual diameter stenosis <50 percent following stenting without in-hospital major adverse cardiovascular events (MACE: composite of cardiac death, myocardial infarction or target vessel revascularization). The primary safety endpoint was freedom from MACE through 30 days follow-up.
The study demonstrated compelling safety and performance results. Shockwave IVL treatment was highly effective in facilitating the delivery of stents and reducing restenosis. Stent deployment was performed in 100 percent of the patients with reduction in residual stenosis to less than 50 percent in all patients despite more than 90 percent of patients having heavily calcified lesions. There were no major intra-procedural complications including perforation, embolization, slow-flow or no reflow and a low MACE rate out to 6 months (8.5 percent). Consistent, reproducible luminal gain was also achieved. The clinical success rate was achieved in 57 (95 percent) patients, limited only by 3 (5 percent) asymptomatic non-Q-wave MI. IVL catheter delivery and treatment at the target lesion was successful in 59 (98.3 percent) patients. The primary safety endpoint of 30-day MACE rates was achieved. There were no cardiac deaths, Q-wave MIs or target vessel revascularizations during this time period.
The Disrupt CAD II study was another a prospective multicenter, single-arm post-approval study conducted at 15 hospitals in 9 countries. Patients with severe CAC with a clinical indication for revascularization underwent vessel preparation for stent implantation with IVL. The primary end point was in-hospital major adverse cardiac events (cardiac death, myocardial infarction, or target vessel revascularization). An optical coherence tomography substudy was performed to evaluate the mechanism of action of IVL, quantifying CAC characteristics and calcium plaque fracture. Independent core laboratories adjudicated angiography and optical coherence tomography, and an independent clinical events committee adjudicated major adverse cardiac events.
In this study, 120 patients were enrolled. Severe CAC was present in 94.2% of lesions. Successful delivery and use of the IVL catheter was achieved in all patients. The post-IVL angiographic acute luminal gain was 0.83±0.47 mm, and residual stenosis was 32.7±10.4%, which further decreased to 7.8±7.1% after drug-eluting stent implantation. The primary end point occurred in 5.8% of patients, consisting of 7 non–Q-wave myocardial infarctions. There was no procedural abrupt closure, slow or no reflow, or perforations. In 47 patients with post-percutaneous coronary intervention optical coherence tomography, calcium fracture was identified in 78.7% of lesions with 3.4±2.6 fractures per lesion, measuring 5.5±5.0 mm in length.
This study demonstrated that in patients with severe CAC who require coronary revascularization, IVL was safe and effective with high procedural success and minimal complications.
Our interventional cardiologists are at the forefront of this technique and regularly treat patients with IVL who have severe calcification in their coronary arteries.